p valence electrons|how to calculate valence electrons : Tagatay Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called .
ako den po makate ang pwerta ko anu pa gamot nito isang linggo napo to lage makate
p valence electrons,Mar 23, 2023 how to calculate valence electrons Learn how to identify and count the valence electrons of different elements, and how they affect chemical reactions and stability. Watch a video explanation and see examples, questions, and comments from other learners.In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope.Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and tips for main group and transition metals. Valence electrons are outer shell electrons that can form chemical bonds with other atoms. Learn how to find the number of valence electrons for main group and transition metals using the .
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called .
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .
Valence electrons are the outer-shell electrons of an atom. Valence electrons determine the reactivity of an atom. Atoms have a tendency to have eight .
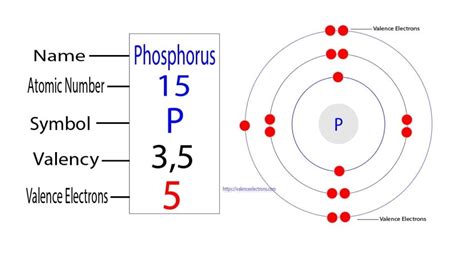
p valence electrons Valence electrons and ionic compounds. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron .p valence electrons how to calculate valence electrons Valence electrons and ionic compounds. When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron . May 19, 2024 So, the valence electrons of calcium are two. The p-block consists of a total of six groups from 13 to 18. The number of maximum valence electrons in the elements of this block is eight and the number of minimum valence electrons is three. The electron configuration of the p-block elements shows that the electron configuration ends in a p .Valence electrons are the s and p electrons in the outermost shell. The electrons present in the inner shell are core electrons. When we study and observe the atom of an element, we come across tiny subatomic . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. The highest-numbered shell is the third shell, which has 2 electrons in the 3s subshell and 3 electrons in the 3p subshell. That gives a total of 5 electrons, so neutral phosphorus atoms have 5 valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons.
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core electrons (1s .
The valence electrons are the electrons that determine the most typical bonding patterns for an element. These electrons are found in the s and p orbitals of the highest energy level for the element. Sodium 1s22s22p63s1. Sodium has 1 valence electron from the 3s orbital. Phosphorus 1s22s22p63s23p3. Phosphorus has 5 valence electrons 2 from the . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.
p valence electrons|how to calculate valence electrons
PH0 · what element has 4 valence electrons
PH1 · valence electrons for transition metals
PH2 · valence electrons chart
PH3 · list of valence electrons for each element
PH4 · how to find valence electrons on pt
PH5 · how to calculate valence electrons
PH6 · how many valence electrons does silver have
PH7 · how many valence electrons does as have
PH8 · Iba pa